
HL Paper 3
The sun is the main source of energy used on earth.
Calculate the energy released, in MeV, in this reaction, using section 36 of the data booklet.
Photovoltaic cells are much less hazardous than nuclear fission.
Early photovoltaic cells were based on silicon containing traces of other elements. State the type of semiconductor produced by doping silicon with indium, In, giving a reason that refers to its electronic structure.
Dye-sensitized solar cells, DSSCs, use a dye to absorb the sunlight. State two advantages that DSSCs have over traditional silicon based photovoltaic cells.
The structure of two dyes used in DSSCs are shown.
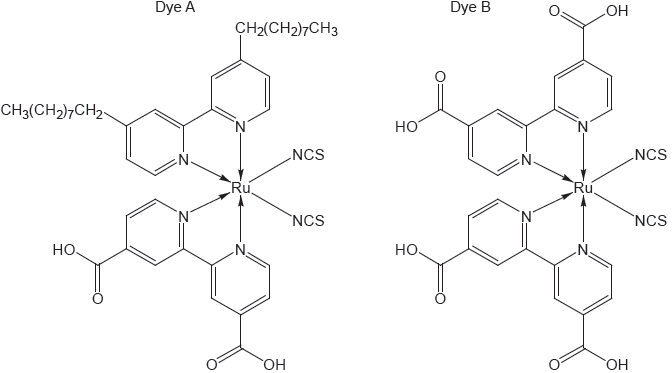
Predict, giving a reason, which dye will absorb light of longer wavelength.
There are many sources of energy available.
Methanol fuel cells provide a portable energy source. The process can be represented by the overall equation CH3OH(aq) + \(\frac{3}{2}\)O2(g) → CO2(g) + 2H2O(g).
Deduce the half-cell equations occurring at each electrode during discharge.
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
Explain how the flow of ions allows for the operation of the fuel cell.
One suggestion for the reduction of carbon footprints is the use of biofuels, such as vegetable oils, as a substitute for petroleum based fuels.
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
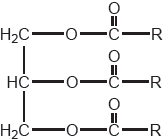
Vegetable oils can be used as a source of energy.
The natural absorption of light by chlorophyll has been copied by those developing dye-sensitized solar cells (DSSCs). Outline how a DSSC works.
Carbon is produced by fusion reactions in stars.
The main fusion reaction responsible for the production of carbon is:
X + \(_2^4{\text{He}} \to _{\;6}^{12}{\text{C}}\)
The mass of X is 8.005305 amu and that of \(_2^4{\text{He}}\) is 4.002603 amu. Determine the energy produced, in J, when one atom of \(_{\;6}^{12}{\text{C}}\) is formed in this reaction. Use section 2 of the data booklet.
\(\beta \)-carotene is involved in the formation of vitamin A. Its sources include carrots, broccoli and dark, leafy vegetables. Its structure is shown below.
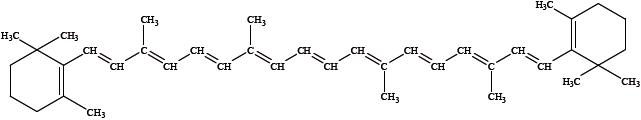
Explain whether\(\beta \)-carotene absorbs ultraviolet or visible radiation.
Nuclear power is another source of energy.
235U atoms can be used in nuclear reactors whereas 238U cannot. A centrifuge is used to separate isotopes.
Calculate the relative rate of effusion of 235UF6(g) to 238UF6(g) using sections 1 and 6 of the data booklet.
Explain, based on molecular structure and bonding, why diffusion or centrifuging can be used for enrichment of UF6 but not UO2.
Traditional photovoltaic cells are made from n-type and p-type semiconductors.
State how n-type and p-type doping of silicon is achieved and the nature of electric charge carriers in each case.
n-type:
p-type:
In dye-sensitized solar cells (DSSCs), nanoparticles coated with a black dye are trapped between electrodes in a liquid electrolyte. Explain the high efficiency of the DSSC structure.
Lead–acid batteries are heavy. Much lighter rechargeable cells are nickel–cadmium batteries used in electronic equipment.
Other than their chemical composition, discuss two major differences between fuel cells and nickel–cadmium cells.
Climate change is a current global topic of debate.
Describe on a molecular level how the greenhouse effect occurs.
Suggest two factors that influence the relative greenhouse effect of a gas.
A fuel cell is an energy conversion device that generates electricity from a spontaneous redox reaction.
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.
State the half-equations for the reactions at both electrodes.
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
Dye-sensitized solar cells (DSSC) convert solar energy into electrical energy.
(i) Describe how a DSSC converts sunlight into electrical energy.
(ii) Explain the role of the electrolyte solution containing iodide ions, I−, and triiodide ions, I3−, in the DSSC.
Describe how silicon may be converted into a p-type semiconductor and explain why this leads to an increase in its electrical conductivit
Nickel-cadmium cells are used to power portable machinery or large tools.
State the equation, including state symbols, for the reaction that takes place when the cell is discharging.
State the physical property of the products that allows this process to be reversed and the cell recharged.
Pure silicon is a semiconductor but its conductivity can be increased when it is doped with small amounts of another element. Describe how the addition of small amounts of arsenic increases the conductivity of silicon.
The concentration of transition metal complexes in water can be determined by visible and ultraviolet (UV-Vis) spectroscopy.
Two octahedral chromium complexes are \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) and \[{{\text{(Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\). Describe how the increase in oxidation state from Cr(II) to Cr(III) and the change in ligand from water to ammonia will affect the splitting of the d orbitals and the frequency of the light these complexes absorb.
One of the following organic compounds is colourless while the other is orange.
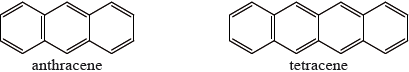
Predict, with reference to conjugation of double bonds, which compound (anthracene or tetracene) will absorb visible light and, therefore, be coloured.
Fuel cells may be twice as efficient as the internal combustion engine. Although fuel cells are not yet in widespread use, NASA has used a basic hydrogen-oxygen fuel cell as the energy source for space vehicles.
State the half-equations occurring at each electrode in the hydrogen-oxygen fuel cell in an alkaline medium.
(+) Cathode:
(–) Anode:
Describe the composition of the electrodes and state the overall cell equation of the nickel-cadmium battery.
(+) Cathode:
(–) Anode:
Cell equation:
Compare a fuel cell and a lead-acid battery, with respect to possible concerns about pollution of the environment.
Although fossil fuels are considered significant sources of energy, the energy conversion associated with the production of electricity is a very inefficient process, often in the region of only 40% of total possible energy conversion.
Fuel cells provide a much more efficient process, often with a 70% conversion factor.
State the energy change conversion involved in a fuel cell.
(i) Identify the two half-equations that take place at the positive electrode (cathode) and negative electrode (anode) in a hydrogen-oxygen fuel cell with an alkaline electrolyte.
Positive electrode (cathode) half-equation:
Negative electrode (anode) half-equation:
(ii) State the overall reaction, identifying the states of all species involved.
(iii) One commercial version of the hydrogen-oxygen fuel cell (with alkaline electrolyte) operates at a temperature of 353 K. The electrodes of the fuel cell are made of graphite but both are covered with a thin layer of platinum. State the function of the platinum.
(iv) Outline the function of the thin polymer membrane used in the corresponding hydrogen-oxygen fuel cell with an acidic electrolyte.
(v) Other than cost, state one disadvantage of a fuel cell.
The photovoltaic cell is a valuable source of energy. Describe its construction and how it responds to sunlight.
Methylene blue can be used as an indicator.
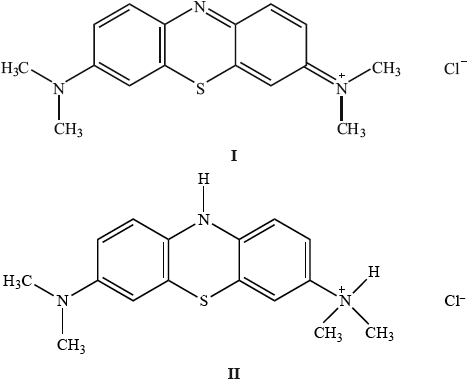
Explain which of the two structures would be coloured.
In terms of the wavelength of the visible light absorbed, suggest why the coloured form is blue.
In the 20th Century, both fission and fusion were considered as sources of energy but fusion was economically and technically unattainable.
Calculate the loss in mass, in kg, and the energy released, in J, when 0.00100 mol of 228Ac decays, each atom losing an electron. Use section 2 of the data booklet and E = mc2.
228Ac → \({}_{ - 1}^0{\text{e}}\) + 228Th
Determine the energy released, in J, by 0.00100 mol of 228Ac over the course of 18 hours.
Outline how nuclear ionising radiation can damage DNA and enzymes in living cells.
Transition metal complexes are coloured because electronic transitions occur within split d orbital energy levels. Identify two different factors that affect the colour of complexes of a specific transition metal.
Phenolphthalein indicator is colourless in solutions with a pH less than 8.2 but pink in solutions with a pH greater than 10.0. The molecule dissociates according to the equation:
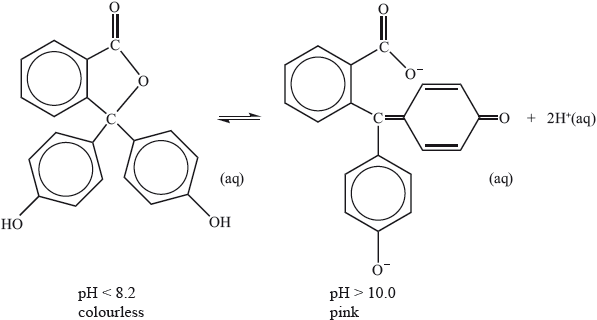
Explain, in terms of the structures, why the indicator is colourless at \({\text{pH}} < 8.2\) and is pink at \({\text{pH}} > 10.0\).
Kevlar® is a lyotropic liquid crystal. Explain the strength of Kevlar® and its solubility in concentrated sulfuric acid.
Describe the use of silicon in photovoltaic cells. Include the following in your description:
• why pure silicon is a better conductor than non-metals such as sulfur and phosphorus
• how a p-type semiconductor made from silicon is different from pure silicon
• how sunlight interacts with semiconductors.
Dye-Sensitized Solar Cells (DSSCs) use organic dyes. Their interaction with light has some similarities to photosynthesis.
Identify two ways in which the structure of the dye shown resembles the chlorophyll molecule. Use section 35 of the data booklet.
Both photosynthesis and the Grätzel cell use energy from sunlight to bring about reduction. Deduce an equation for the reduction reaction in the electrolyte of a Grätzel cell.
Crude oil is a useful energy resource.
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
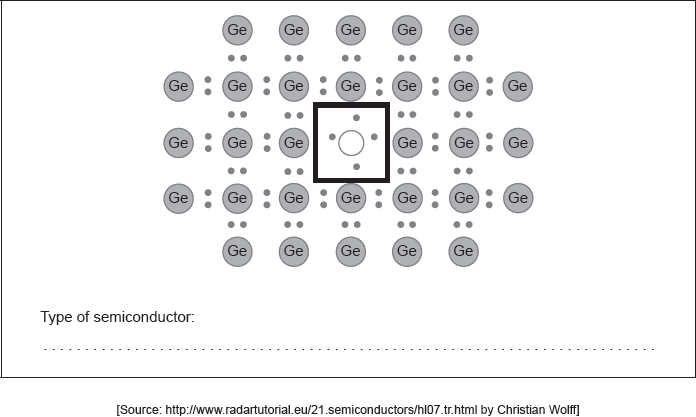
The conductivity of a germanium semiconductor can be increased by doping.
A dye-sensitized solar cell uses a ruthenium(II)–polypyridine complex as the dye. Two ruthenium(II) complexes, A and B, absorb light of wavelengths 665 nm and 675 nm respectively.
Draw the Lewis (electron dot) structure for an appropriate doping element in the box in the centre identifying the type of semiconductor formed.
State the feature of the molecules responsible for the absorption of light.
Outline why complex B absorbs light of longer wavelength than complex A.
The combustion of fossil fuels produces large amounts of CO2, a greenhouse gas.
The diagram below illustrates a range of wavelengths in the electromagnetic spectrum.
The structures of 11-cis-retinal and β-carotene are given in section 35 of the data booklet. Suggest a possible wavelength of light absorbed by each molecule using section 3 of the data booklet.
A fuel cell converts chemical energy directly to electrical energy.
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
Modern electric cars store their energy in lithium ion batteries.
The diagram represents a cell in such a battery delivering a current.
The carbon footprint of electric cars depends on how the electricity is produced. Nuclear fission of 235U is one source of electrical energy that has a minimal carbon footprint.
Complete the half-equations on the diagram and identify the species moving between the electrodes.
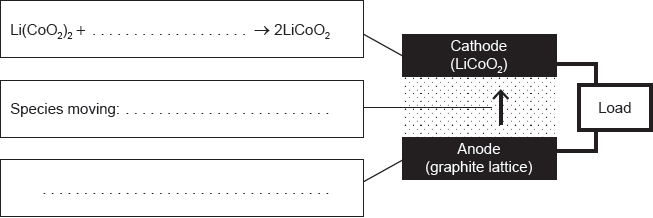
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
Explain how the proportion of 235U in natural uranium is increased.
Fuel cells and rechargeable batteries are useful sources of energy.
One type of fuel cell contains a proton exchange membrane between electrodes and uses aqueous methanol as the fuel.
State half-equations for the reactions which occur at the negative and positive electrodes.
Negative electrode (anode):
Positive electrode (cathode):
Suggest one advantage and one disadvantage of a fuel cell over a lead–acid battery as an energy source in a motor vehicle.
Advantage:
Disadvantage:
As well as being burnt, methanol can also be used to provide electricity through a fuel cell. A schematic diagram of such a fuel cell, that depends on the transfer of hydrogen ions between the electrodes, is shown below.
Even though fuel cells, primary cells and rechargeable cells have similar fundamental characteristics, there are important differences between them.
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
Suggest a way in which they are similar.
Outline the difference between primary and rechargeable cells.
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
A Grätzel dye-sensitized solar cell (DSSC) and a silicon based photovoltaic cell both convert solar energy into electrical energy by producing a charge separation.
Contrast how absorption of photons and charge separation occur in each device.
Suggest one advantage a DSSC has over a silicon based photovoltaic cell.